Introduction
Spontaneous intestinal perforation (SIP) is an isolated focal intestinal perforation usually 5-10 mm with histologic findings of hemorrhagic necrosis of the antimesenteric border of the distal ileum with the absence of inflammation, ischemia, or coagulation necrosis, clinically distinct from necrotizing enterocolitis (NEC). [1], [2] SIP occurs most often in the first week of life in extremely low birth weight (ELBW) < 1,000-gram neonates with an incidence of 3-8% [3], [4], [5] , in contrast to a later onset of NEC in preterm neonates. Although SIP has better clinical outcomes than intestinal perforation secondary to NEC, mortality remains high (20-40%). [2], [3], [6] Additionally, SIP has an association with poor neurodevelopmental outcomes at 18-22 months, corrected age. The specific etiology of NEC and SIP remains elusive. Both can present acutely with few antecedent signs. With feeding protocols and exclusive use of breast milk, the incidence of NEC is not increasing. With smaller and smaller neonates being resuscitated, SIP is projected to become the most common form of surgical bowel disease in extremely low birth weight (ELBW) neonates.
Studies have demonstrated multiple risk factors for the development of SIP, such as postnatal corticosteroids, indomethacin, chorioamnionitis, and patent ductus arteriosus (PDA), however, no one specific biomarker has been found to predict NEC or SIP. [2], [4] The pathophysiology of SIP and NEC is not fully understood, but it appears that the underlying disease mechanisms are different for both. [6], [7] Infection, inflammatory response, ischemia, immunologic barrier dysfunction, and endothelial injury are postulated to play roles in NEC. [7], [8], [9] Animal models have demonstrated possible mechanisms for SIP, including steroid-induced bowel wall submucosal thinning with mucosal hyperplasia and perturbations in nitric oxide metabolism from altered blood flow. [1] The ileum may be particularly vulnerable to mucosal hyperplasia as there is a concentration of insulin-like growth factor-1 within the mesenchymal tissue of the neonatal ileum that is displaced after exposure to steroids. In addition, studies have shown a loss of the anti-apoptotic factor, transforming growth factor alpha, in the muscularis externa of neonatal mice after dexamethasone treatment that may allow the necrosis of smooth muscle seen in SIP. [1]
SIP can be clinically challenging to differentiate from intestinal perforation secondary to NEC.[6] In contrast to NEC, the intestinal mucosa of SIP will have no evidence of inflammation or pneumatosis. SIP is more commonly seen in very low birth weight (VLBW) < 1,500 grams and ELBW neonates who have a PDA treated with indomethacin, have received vasopressor support, or have received hydrocortisone. [1], [3], [4] There is rarely associated physiologic deterioration with a usual presentation of abrupt onset of abdominal distention with discoloration and a gasless abdomen without pneumatosis intestinalis or portal venous air. [2] On the other hand, NEC presents on average at 2-3 weeks of life and often at 4-6 weeks in ELBW neonates.[7] Unlike SIP, neonates with NEC frequently develop leukopenia, thrombocytopenia, hyponatremia, metabolic acidosis, and glucose instability.[7] For cases of SIP with single perforation, peritoneal needle aspiration or placement of Penrose drains is the treatment of choice. [3], [6], [12]
It is important to differentiate SIP from NEC to identify appropriate management and outcomes. In view of its unpredictable onset and high mortality, research has been conducted on NEC biomarkers with a special focus on inflammatory mediators and on the gut microbiome of preterm neonates. [11], [13], [14] Some studies have identified a reduced stool microbial diversity in preterm versus term neonates, leading to the hypothesis of “inappropriate colonization” precipitating NEC. [14], [21] There is insufficient data to discern SIP from NEC using biomarkers. By identifying a biomarker, we could recognize a population to study putative interventions to potentially prevent SIP.
Nucleated red blood cells (NRBC) are immature erythrocytes produced in the fetal bone marrow that are released in response to hypoxia and possibly inflammatory mediators. [15], [16] An association between hypoxia and an increased NRBC count has been noted in animal models and in human neonates. Although it is uncommon to find NRBCs in the peripheral blood in other age groups, NRBC counts up to 4/100 WBC are considered normal on the first day of life. Initial neonate NRBC counts can be elevated at birth with perinatal stress such as intrauterine growth restriction (IUGR), maternal hypertension, chorioamnionitis, multiple births, congenital anemia, or difficult transition to extrauterine life with depressed APGAR scores.[17], [18], [19], [20] We hypothesized that uteroplacental insufficiency reflected in neonate NRBC at birth could be a risk factor for gastrointestinal disease in neonates.
Methods
After obtaining institutional review board approval, analysis was conducted using a retrospective chart review of neonates admitted to the fifty-bed level III neonatal intensive care unit at Loyola University Medical Center between the years of 2008 to 2018. Premature neonates with a clinical diagnosis of SIP were compared to premature neonates with stage II and stage III NEC and control premature neonates without SIP or NEC of matched gestational age (GA) and birth weight (BW). [10] Neonates with congenital cardiac, pulmonary, gastrointestinal, and chromosomal abnormalities, congenital anemia, and a birth GA of 37 weeks or greater were excluded from the study.
Kruskal-Wallis test, Chi-Square test, or Fisher's exact test were used to compare distributions of baseline characteristics between groups and perinatal stress characteristics between groups. Univariate and multivariate nominal logistic regression models were used to estimate the association of baseline NRBC between groups; multivariable models were adjusted for BW and GA. Given the skewness of NRBCs, nominal logistic regression models used a log transformation of NRBC to detect differences in unadjusted and adjusted outcomes. Median times to SIP and NEC were calculated using the Kaplan-Meier method of each subset. Longitudinal comparisons of NRBC were done cross-sectionally up to week 4 using the Kruskal-Wallis test at each timepoint given enough variation; otherwise not calculated (i.e. if < 75% with a value of zero, not calculated). An NRBC value of < 4 was considered normal. The Chi-square test or Fisher's exact test was used to compare frequencies of abnormal NRBC cross-sectionally up to week 4. Significance was defined by 2-sided p-values < 0.05. All analyses were performed with SAS 9.4 (Cary, NC).
Results
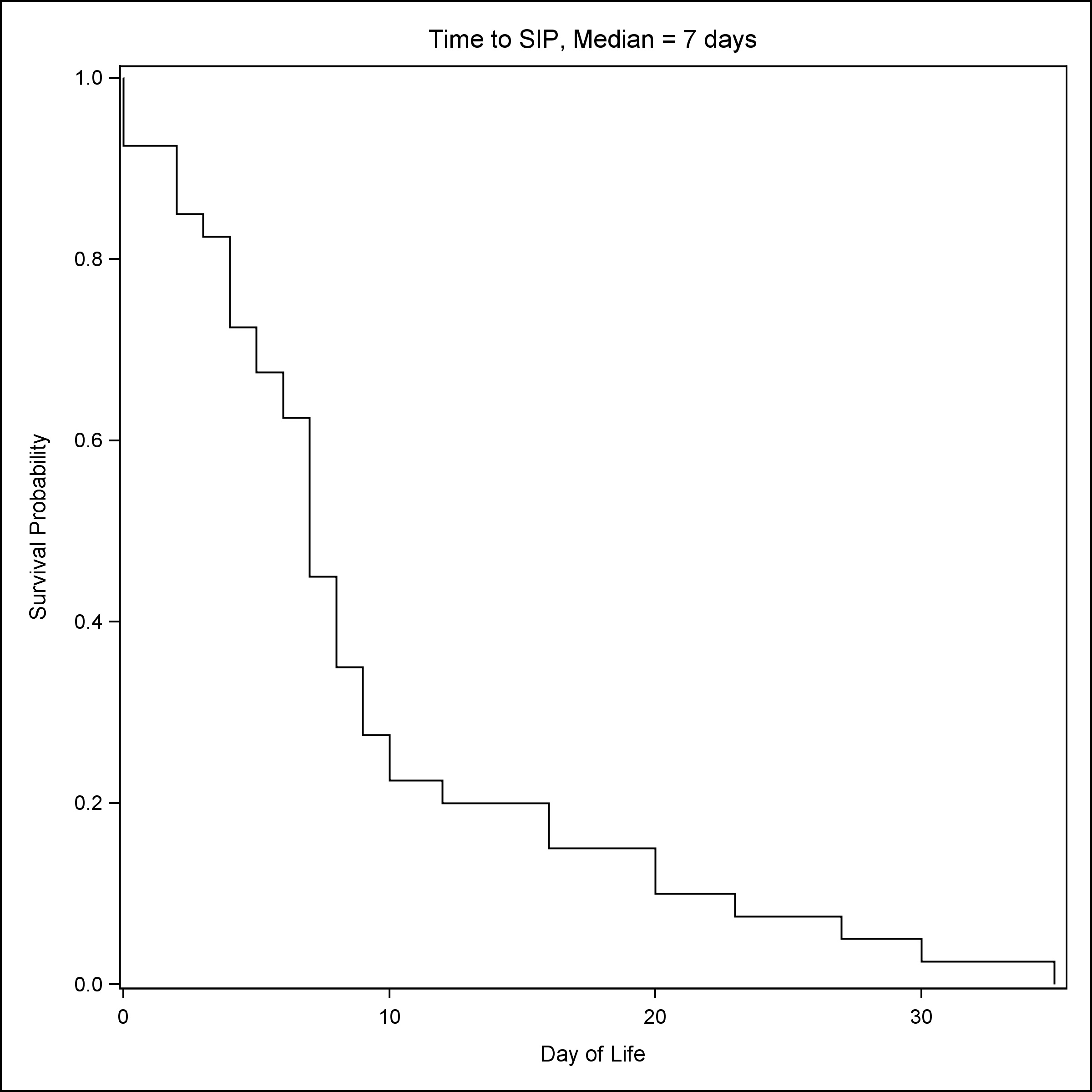
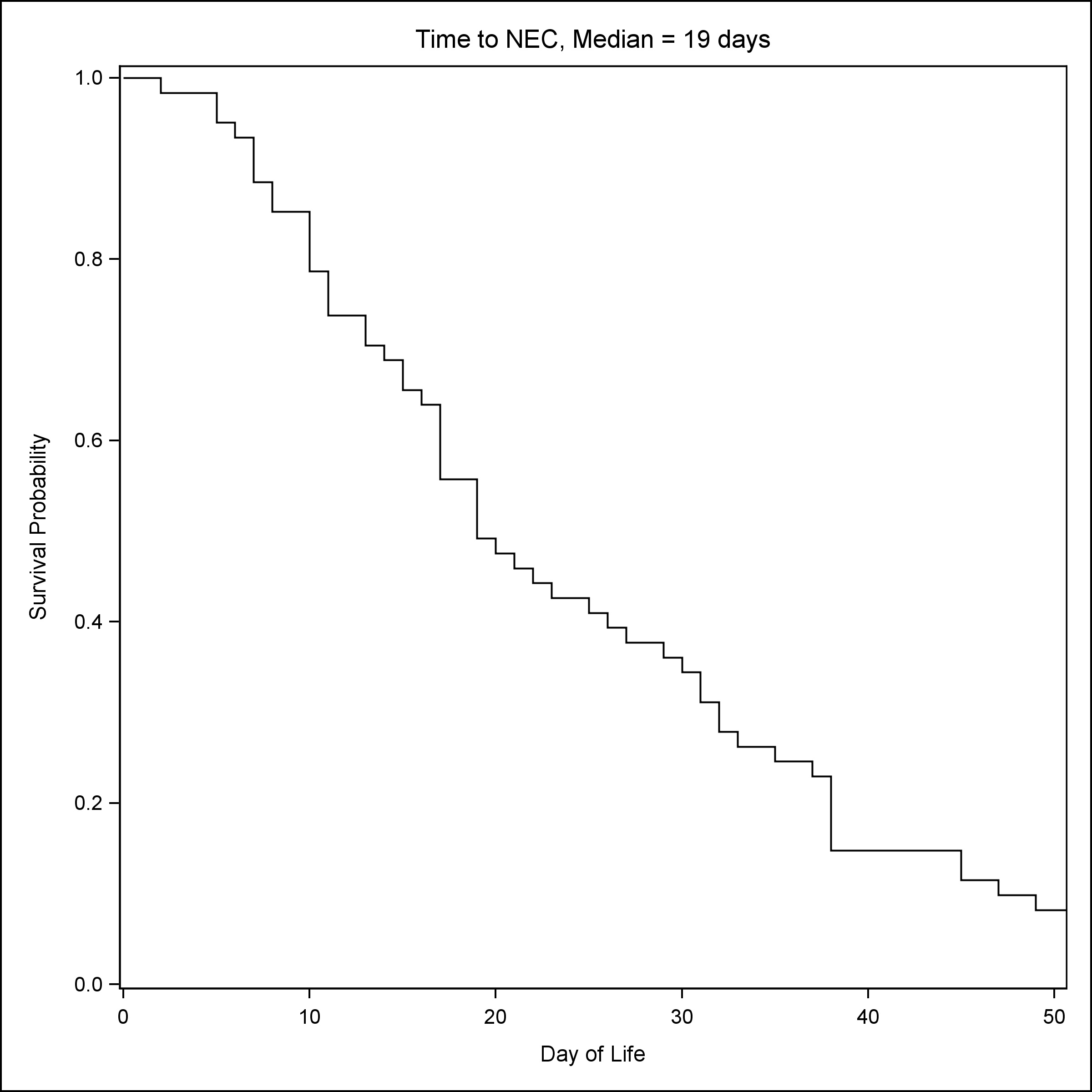
A total of 239 neonates were included in the study, 40 had a clinical diagnosis of SIP, 61 had stage II or stage III NEC, and 138 neonates were controls matched for GA and BW. As compared to NEC patients, patients with SIP were more likely to be male (72.5% vs 47.5%) and had lower GA [Median (Q1, Q3): 25.1 (23.8, 28) vs 27 (25.1, 31.1)] and lower BW [Median (Q1, Q3): 690 (585, 1072) vs 960 (680, 1560)] (Table 1) . NRBCs at birth were the lowest in groups that later developed NEC [Median (Q1, Q3): NRBC count 10/100 WBC: 9 (5, 29)] (Table 1). Consistent with established reports, the median time to SIP was seven days (Fig. 1), whereas the median time to NEC was 19 days (Fig. 2).
Elevated baseline NRBC was associated with decreased odds of developing NEC compared to controls [OR: 0.70 (0.55, 0.89), p-value=0.0033] (Table 2). Elevated baseline NRBC was associated with increased odds of SIP relative to NEC [OR: 1.61 (1.18, 2.20), p-value=0.0027](Table 2). There were no statistical differences in perinatal stresses of IUGR, maternal hypertension, chorioamnionitis, multiple births, or depressed APGAR scores between the three study groups (Table 3).
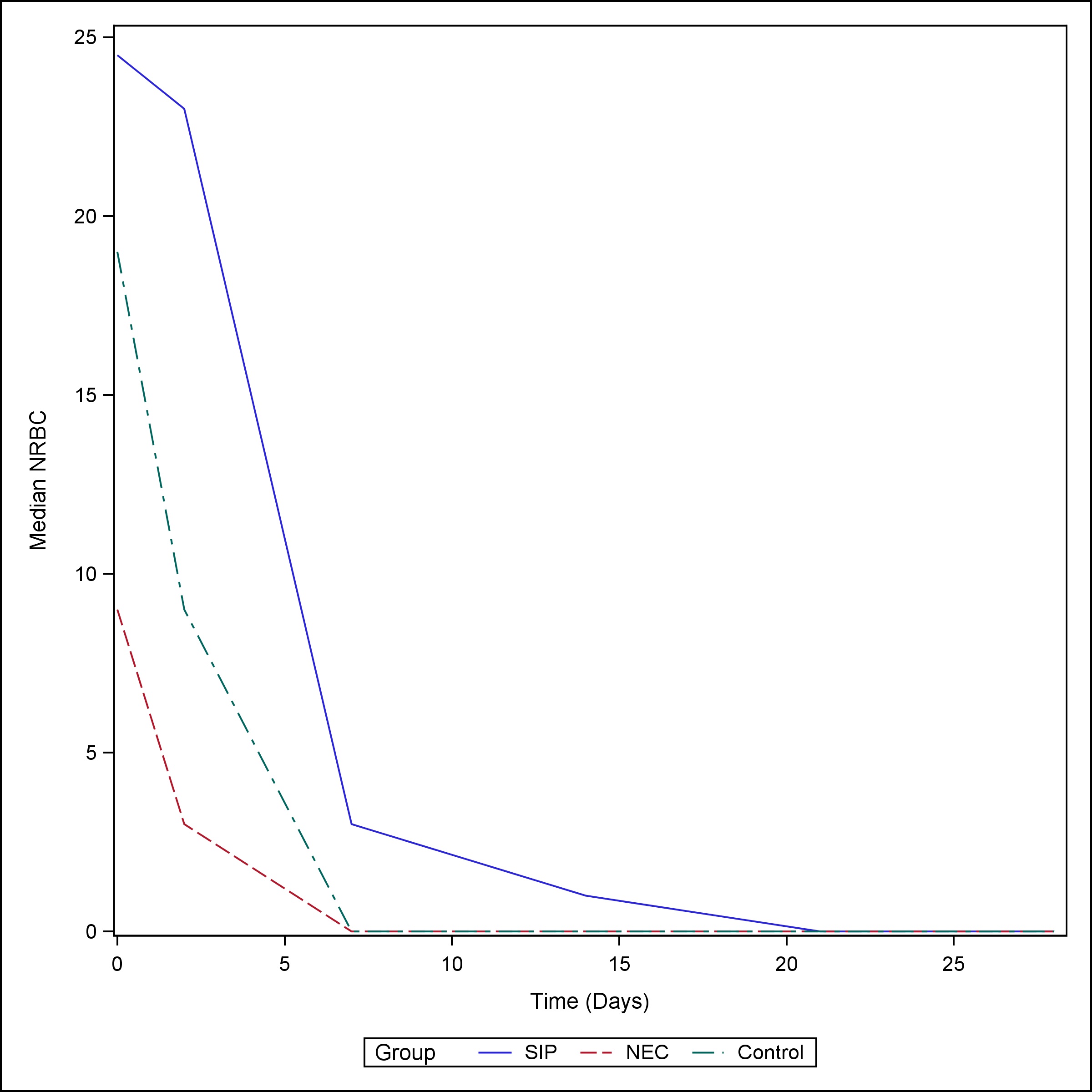
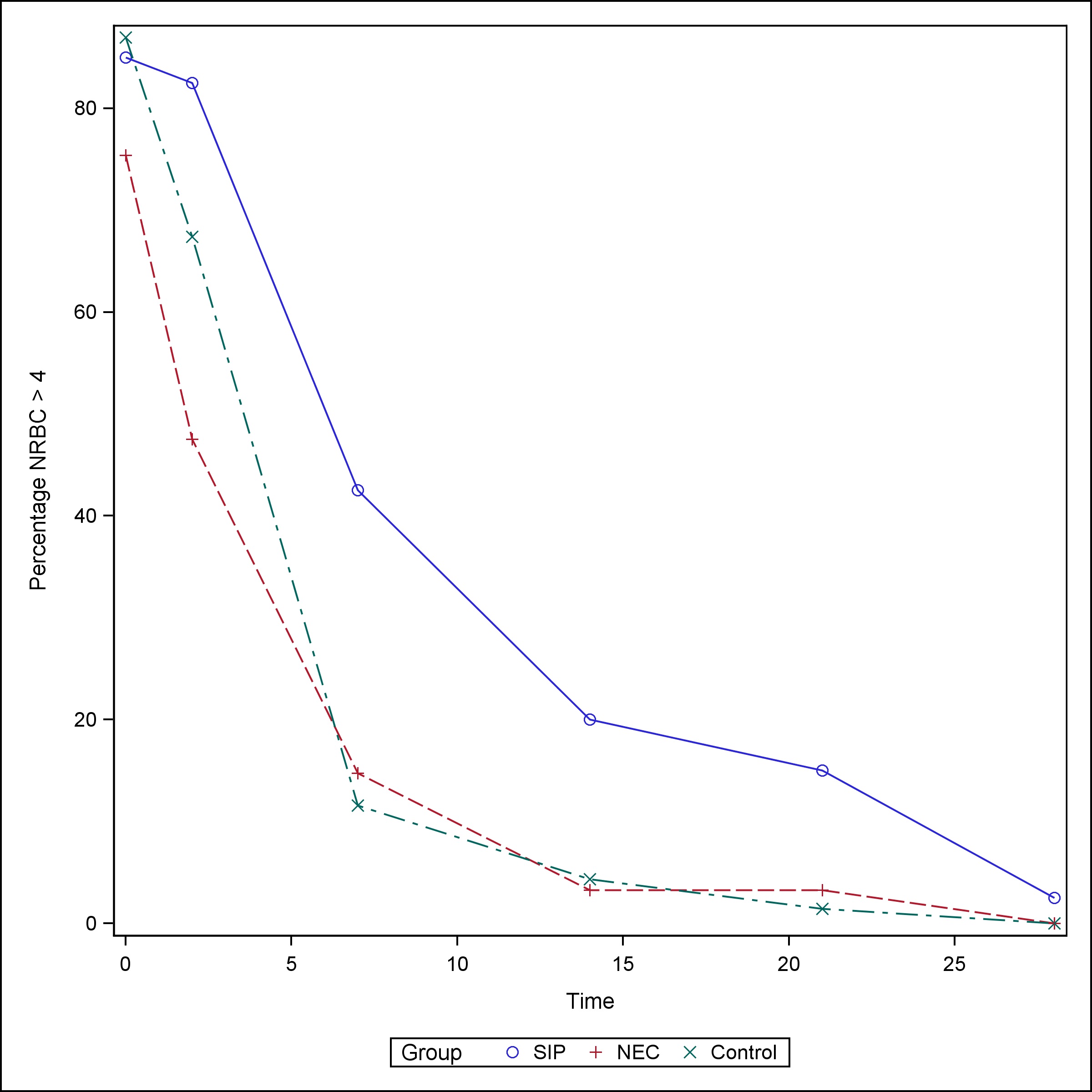
The median NRBC value at birth for neonates with SIP was 24.5/100 WBC (Q1, Q3: 6, 93) and remained elevated up to the third day of life [Median (Q1, Q3): 23 (6, 93)]. NEC neonates had a medium NRBC value of 9/100 WBC (Q1, Q3: 5, 29) at birth and normalized to less than 4/100 WBC by day of life (DOL) 3. SIP neonates continued to have a median NRBC > 1 at week one of life, whereas NEC and control neonates had median NRBC values of 0 starting at one week of life (Table 4), (Fig. 3)). The proportion of neonates with elevated NRBC count (>4/100 WBC) did not vary at birth between the groups. However, the proportion of SIP patients with elevated NRBC counts was higher than both the NEC patients and controls on DOL 1-3 (82.5% vs 47.6% vs 67.4%, p-value = 0.0011), week 1 (42.5% vs 14.8% vs 11.6%, p-value =0.0011), week 2 (20% vs 3.3% vs 4.4%, p-value = 0.0033), and week 3 (15% vs 3.3% vs 1.5%, p-value = 0.0124) (Table 5), (Fig. 4).
Discussion
In this study, we report that NRBCs are elevated shortly after birth and in the first 3 days of life in preterm neonates who go on to develop SIP. Elevated NRBC at birth with delayed normalization is characteristic of chronic hypoxic ischemic compromise. [22] A unique finding in our study is that the NRBC count in SIP neonates took longer to normalize than in premature NEC and control neonates. The increase and delayed normalization of NRBC in the SIP group is consistent with more prolonged uteroplacental insufficiency and diving reflex.
The role of NRBC as a prognostic biomarker in neonates has been previously studied. An increase in NRBC count can be seen in asphyxia, where the degree of NRBC elevation related to the duration and severity of asphyxia has been explored. Boskabadi et. al. demonstrated that there was 94% predictive power when NRBC count was used in combination with hypoxic-ischemic encephalopathy grading to determine the prognosis of asphyxiated neonates. [15]
Surgical findings in SIP are reasonably typical and constant. One or two perforations are found in the terminal ileum, on the antimesenteric border, in otherwise normal appearing bowel. Pathology on resected specimens frequently shows hemorrhagic necrosis. This is in contrast to the ischemic and coagulative necrosis seen in NEC. Thinning of the submucosa and muscularis is also seen at the site of perforation. There are also many physiological alterations present in the intestine of the premature newborn, including decreased motility that improves with advancing gestation. We hypothesize that anatomic and physiological immaturity of the ELBW's gastrointestinal tract can become vulnerable and exacerbated by fetal hypoperfusion reflected by elevated NRBCs at birth with delayed normalization. Male gender vulnerability has been previously demonstrated in neonates born prematurely. Compared to females, males have more postnatal complications including lower Apgar scores, higher rates of respiratory distress syndrome, and higher perinatal mortality rates. [23] It is certainly feasible that the gastrointestinal tract of the male is more immature than their female counterparts increasing the vulnerability to SIP. This vulnerability is then exacerbated by the stress of hypoperfusion, as evidenced by sustained elevation of NRBC.
Our study has several limitations. This was a retrospective study and laboratory values were not obtained at consistent times, so they evaluated by date ranges to follow up NRBC after the initial birth labs were drawn. Although different entities, SIP like NEC most likely has a multifactorial etiology. Such risk factors include hypoxia, hypoperfusion, cord and neonate pH, use of steroids, use of NSAIDs, intraventricular hemorrhage, and delayed enteral feeding. Although prospective studies are needed, our data demonstrate that a premature male neonate with elevated NRBC at birth is at significant risk for developing SIP.
We hypothesize that chronic uteroplacental insufficiency would result in the redistribution of cardiac output through the primitive diving reflex from the negotiable circulation of the gut to the non-negotiable circulation of the heart, brain, and adrenal glands, ultimately making the small intestine more vulnerable to non-inflammatory perforation. The potential interactions between perinatal hypoxic-ischemic compromise, endogenous steroids, and gut mucosal integrity need further investigation.
Conclusion
This study provides new information about NRBC as a potential biomarker for SIP. Our data show that elevated baseline NRBCs were associated with increased odds of developing SIP compared to NEC. It demonstrates a significant association of SIP in ELBW males with elevated NRBCs on admission and continued elevation on DOL 1-3. Based on our study, any ELBW male with elevated NRBC at birth requires vigilance and judicious advancement of feeds in the first week of life.
Figures
|
|
|
|
|
Figure 1
Time to SIP.
|
|
|
|
Figure 2
Time to NEC.
|
|
|
|
Figure 3
Median NRBC by the group over time.
|
|
|
|
Figure 4
Percentage with NRBC > 4.
|
Tables
[TableWrap ID: t1
]
Table 1
Descriptive Statistics, n (%)
|
Spontaneous bowel perforation (SIP)N=40
|
Necrotizing enterocolitis (NEC)N=61
|
ControlsN=138
|
P value* |
| Gender |
|
|
|
0.0402 |
| Male |
29 (72.5%) |
29 (47.54%) |
74 (53.62%) |
|
| Female |
11 (27.5%) |
32 (52.46%) |
64 (46.38%) |
|
| Race |
|
|
|
0.0561 |
| Caucasian |
15 (37.5%) |
20 (32.79%) |
56 (40.58%) |
|
| African American |
6 (15.0%) |
18 (29.51%) |
37 (26.81%) |
|
| Hispanic |
18 (45.0%) |
20 (32.79%) |
30 (21.74%) |
|
| Asian |
1 (2.5%) |
2 (3.28%) |
4 (2.9%) |
|
| Others |
0 (0%) |
1 (1.64%) |
11 (7.97%) |
|
| Gestational age, Median (Q1, Q3) |
25.1 (23.8, 28) |
27 (25.2, 31.1) |
27 (25.2, 27.2) |
0.0051 |
| Birth weight, Median (Q1, Q3) |
690 (585, 1072) |
960 (680, 1560) |
860 (730, 1040) |
0.0122 |
| Admission NRBC |
24.5 (7, 104) |
9 (5, 29) |
19 (10, 51) |
0.0064 |
| Hematocrit at birth |
42.95 (36.45, 46.45) |
43 (35.25, 46) |
43.15 (5.76) |
0.385 |
| Indomethacin |
|
|
|
0.0294 |
| Yes |
8 (20.0%) |
9 (15%) |
44 (31.88%) |
|
| No |
32 (80.0%) |
51 (85%) |
94 (68.12%) |
|
| Ibuprofen |
|
|
|
0.1258 |
| Yes |
10 (25.0%) |
14 (23.33%) |
19 (13.77%) |
|
| No |
30 (75.0%) |
46 (76.67%) |
119 (86.23%) |
|
| Postnatal steroids |
|
|
|
<0.0001 |
| Hydrocortisone |
16 (40.0%) |
6 (10%) |
8 (5.8%) |
|
| Dexamethasone |
4 (10.0%) |
4 (6.67%) |
9 (6.52%) |
|
| None |
20 (50.0%) |
50 (83.33%) |
121 (87.68%) |
|
| Vasopressors |
|
|
|
<0.0001 |
| Dopamine |
17 (42.5%) |
13 (21.67%) |
25 (18.12%) |
|
| Dobutamine |
3 (7.5%) |
2 (3.33%) |
13 (9.42%) |
|
| Epinephrine |
11 (27.5%) |
2 (3.33%) |
6 (4.35%) |
|
| IVH |
|
|
|
0.0224 |
| Yes |
19 (31.67%) |
15 (20%) |
36 (20.69%) |
|
| No |
21 (68.33%) |
45 (80%) |
102 (79.31%) |
|
* P-value calculated with Kruskal-Wallis test, Chi-square test, or Fisher’s exact test, as appropriate.
[TableWrap ID: t2
]
Table 2
Association of baseline log (NRBC), per log unit increase
| Response |
Odds Ratio Unadjusted**(confidence interval)
|
P-Value |
Odds Ratio adjusted** (confidence interval) |
P-Value |
| SIP vs. Control |
1.13 (0.87, 1.47) |
0.3609 |
1.33 (0.96, 1.84) |
0.0887 |
| NEC vs. Control |
0.70 (0.55, 0.89)* |
0.0033 |
0.76 (0.58, 0.99)* |
0.0398 |
| SIP vs. NEC |
1.61 (1.18, 2.20)* |
0.0027 |
1.75 (1.20, 2.55)* |
0.0035 |
**Odds ratio and p-value estimated using a nominal logistic regression model. The adjusted model included covariates of gestational age and birth weight.
[TableWrap ID: t3
]
Table 3
Characteristics contributing to perinatal stress
|
Spontaneous bowel perforation (SIP)N=40
|
Necrotizing enterocolitis (NEC)N=61
|
ControlsN=138
|
P value* |
| IUGR, n (%) |
2 (5%) |
4 (7%) |
11 (8%) |
0.940 |
| Maternal HTN, n (%) |
2 (5%) |
8 (13%) |
9 (7%) |
0.236 |
| Chorioamnionitis, n (%) |
4 (10%) |
9 (15%) |
12 (9%) |
0.390 |
| Preeclampsia, n (%) |
4 (10%) |
6 (10%) |
20 (14%) |
0.588 |
| Multiple Births, n (%) |
12 (30%) |
12 (20%) |
38 (28%) |
0.412 |
| 1 min Apgar, Median (Q1, Q3) |
5 (2, 7) |
6 (3, 8) |
6 (4, 7) |
0.252 |
| 5 min Apgar, Median (Q1, Q3) |
7.5 (4, 9) |
8 (7, 9) |
8 (7, 9) |
0.253 |
| 10 min Apgar, Median (Q1, Q3) |
8 (7, 9) |
8 (8, 9) |
8 (8, 9) |
0.468 |
*P-value calculated with Kruskal-Wallis test, Chi-square test, or Fisher’s exact test, as appropriate.
[TableWrap ID: t4
]
Table 4
Median (Q1, Q3) of NRBC by DOL
| Group |
Day 0 |
Day 1-3 |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
| SIP |
24.5 (7, 104), N=40 |
23 (6, 93), N=40 |
3 (0, 15), N=40 |
1 (0, 4), N=40 |
0 (0, 1.5), N=40 |
0 (0,0), N=40 |
| NEC |
9 (5, 29), N=61 |
3 (0, 18), N=61 |
0 (0, 2), N=61 |
0 (0, 2), N=61 |
0 (0, 0), N=61 |
0 (0, 0), N=61 |
| Control |
19 (10, 51), N=138 |
9 (3, 270, N=138 |
0, (0, 2), N=168 |
0 (0, 0), N=168 |
0 (0, 0), N=168 |
0 (0, 0), N=168 |
| P-Value* |
0.0064 |
0.0020 |
NA |
NA |
NA |
NA |
*P-Value calculated with Kruskal Wallis test
[TableWrap ID: t5
]
Table 5
The proportion of individuals with NRBC greater than 4
| Group |
Day 0 |
Day 1-3 |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
| SIP |
34/40 (85%) |
33/40 (82.5%) |
17/40 (42.5%) |
8/40 (20%) |
6/40 (15%) |
1/40 (2.5%) |
| NEC |
46/61 (75%) |
29/61 (47.5%) |
9/61 (14.8%) |
2/61 (3.3%) |
2/61 (3.3%) |
0/61 (0%) |
| Control |
120/138 (87%) |
93/138 (67.4%) |
16/138 (11.6%) |
6/168 (4.4%) |
2/138 (1.5%) |
0/138 (0%) |
| P-Value* |
0.1230 |
0.0010 |
0.0010 |
0.0036 |
0.0024 |
0.1674 |
*P-Value calculated with chi-square test or Fisher’s exact test, as appropriate.
Notes
n1Abbreviations
SIP: Spontaneous intestinal perforation
NEC: necrotizing enterocolitis
ELBW: extremely low birth weight
PDA: patent ductus arteriosus
VLBW: very low birth weight
NRBC: nucleated red blood cells
WBC: white blood cells
IUGR: intrauterine growth restriction
GA: gestational age
BW: birth weight
DOL: day of life
n2Conflicts of interest. None.
n4Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n5Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
References
|
|
| 1. |
Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight neonate. Pediatr Res. 2009; 65:138-44. Available from: https://doi.org/10.1203/PDR.0b013e31818c7920. |
| 2. |
Shah J, Singhal N, Da Silva O, Rouvinez-Bouali N, Seshia M, Lee SK, et al. Intestinal perforation in very preterm neonates: Risk factors and outcomes. J Perinatol. 2015; 35: 595-600. Available from: https://doi.org/10.1038/jp.2015.41. |
| 3. |
Adant I, Miserez M, Naulaers G, Carkeek K, Ortibus E, Aerts R, et al. Long-term outcomes of very low birth weight neonates with spontaneous intestinal perforation: A retrospective case-matched cohort study. J Pediatr Surg. 2019; 54: 2084-91. Available from: https://doi.org/10.1016/j.jpedsurg.2019.04.012.
|
| 4. |
Ragouilliaux CJ, Keeney SE, Hawkins HK, Rowen JL. Maternal factors in extremely low birth weight neonates who develop spontaneous intestinal perforation. Pediatr. 2007; 120:1458-64. Available from: https://doi.org/10.1542/peds.2006-2804.
|
| 5. |
Wadhawan R, Oh W, Vohr BR, Saha S, Das A, Bell EF, et al. Spontaneous intestinal perforation in extremely low birth weight neonates: association with indomethacin therapy and effects on neurodevelopmental outcomes at 18-22 months corrected age. Arch Dis Child Fetal Neonatal Ed. 2013; 98:127-32. Available from: https://doi.org/10.1136/archdischild-2011-300659.
|
| 6. |
6. Tiwari C, Sandlas G, Jayaswal S, Shah H. Spontaneous intestinal perforation in neonates. J Neonatal Surg. 2015; 4:14. Available from: https://doi.org/10.21699/jns.v4i2.201.
|
| 7. |
Kampanatkosol R, Thomson T, Habeeb O, Glynn L, Dechristopher PJ, Yong S, et al. The relationship between reticulated platelets, intestinal alkaline phosphatase, and necrotizing enterocolitis. J Pediatr Surg. 2014; 49: 273-6. Available from: https://doi.org/10.1016/j.jpedsurg.2013.11.037.
|
| 8. |
Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: Necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011; 158:403-9. Available from: https://doi.org/10.1016/j.jpeds.2010.09.015.
|
| 9. |
Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: Past, present, and future. Clin Perinatol. 2013; 40: 27-51. Available from: https://doi.org/10.1016/j.clp.2012.12.012.
|
| 10. |
Bhatia AM, Stoll BJ, Cismowski MJ, Hamrick SE. Cytokine levels in the preterm neonate with neonatal intestinal injury. Am J Perinatol. 2014; 31:489-96. Available from: https://doi.org/10.1055/s-0033-1353437.
|
| 11. |
Chan KYY, Leung FWL, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm neonates. Plos one. 2012; 7:36977. Available from: https:/doi.org/10.1371/journal.pone.0036977.
|
| 12. |
U.S. National Library Library of Medicine, National Institute of Health. Available from: https://www.nichd.nih.gov/sites/default/files/about/Documents/Nest_Protocol.pdf.
|
| 13. |
Ng PC, Chan KYY, Poon TCW. Biomarkers for prediction and diagnosis of necrotizing enterocolitis. Clin Perinatol. 2013; 40:149-59. Available from: https://doi.org/10.1016/j.clp.2012.12.005. |
| 14. |
Rusconi B, Good M, Warner BB. The Microbiome and biomarkers for necrotizing enterocolitis: Are we any closer to prediction? J Pediatr. 2017; 189:40-7. Available from: https://doi.org/10.1016/j.jpeds.2017.05.075. |
| 15. |
Boskabadi H, Zakerhamidi M, Sadeghian MH, Avan A, Ghayour-Mobarhan M, Ferns GA. Nucleated red blood cells count as a prognostic biomarker in predicting the complications of asphyxia in neonates. J Matern Neonatal Med. 2017; 30:2551-6. Available from: https://doi.org/10.1080/14767058.2016.1256988. |
| 16. |
Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008; 198:426. Available from: https://doi.org/10.1016/j.ajog.2008.01.040. |
| 17. |
Christensen RD, Lambert DK, Richards DS. Estimating the nucleated red blood cell “emergence time” in neonates. J Perinatol. 2014; 34:116-9. Available from: https://doi.org/10.1038/jp.2013.113. |
| 18. |
Cremer M, Roll S, Gräf C, Weimann A, Bührer C, Dame C. Nucleated red blood cells as marker for an increased risk of unfavorable outcome and mortality in very low birth weight neonates. Early Hum Dev. 2015; 91:559-62. Available from: https://doi.org/10.1016/j.earlhumdev.2015.06.004. |
| 19. |
Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001; 84:211-5. Available from: https://doi.org/10.1136/fn.84.3.f211. |
| 20. |
Ravishankar V, Buhimschi CS, Booth CJ, Bhandari V, Norwitz E, Copel J, et al. Fetal nucleated red blood cells in a rat model of intrauterine growth restriction induced by hypoxia and nitric oxide synthase inhibition. Am J Obstet Gynecol. 2007; 196:482. Available from: https://doi.org/10.1016/j.ajog.2006.12.020. |
| 21. |
Wang K, Guozhong T, Zhen S, Sylvester KG. Recent potential noninvasive biomarkers in necrotizing enterocolitis. Gastroent Res Pract. 2019; 8413698. Available from: https://doi.org/10.115/2019/8413698. |
| 22. |
Dina P, Muraskas JK. Hematologic changes in newborns with neonatal encephalopathy. Neoreviews. 2018; 19:29-33. Available from: https://doi.org/10.1542/neo.19-1-e29. |
| 23. |
American College of Obstetricians and Gynecologists. Neonatal Encephalopathy and Neurologic Outcome, Second Edition. Obstetrics and Gynecology. 2014; 7:121. |